

 |
 |
 |
 |
 |
Shenzhen-Hong Kong Consortium Successfully Developed SARS Hyperimmune Globulins (Human) for the Treatment of Patients of Future SARS Outbreaks ˇV A Completed National 863 Project
Hong Kong (July 27, 2003) ˇV Shenzhen WeiWu Guangming Biological Products Company Limited and Advantek Biologics Limited (a Hong Kong Biotechnology Company) announced today that safe, top quality, clinical-grade SARS hyperimmune globulins (human) had been successfully developed for the treatment of patients of future SARS outbreaks. The SARS hyperimmune globulins development project is a successfully completed SARS-related 863 Programme commissioned by the Chinese State Council Ministry of Science and Technology. Since the SARS hyperimmune globulins are prepared from human convalescent plasma and processed with virus-inactivation technologies recognized by authorities worldwide (including the US FDA), the risk of allergic reactions and potential viral infection is considered minimal.
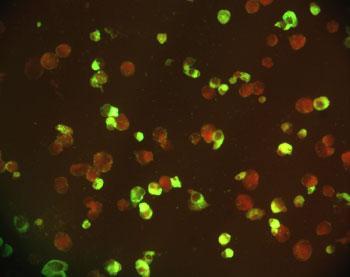
Hyperimmune globulins are immunoglobulins (or called antibodies) purified from convalescent plasma (plasma of recovered patients). Immunoglobulins (comprised ~90% of IgG and ~10% of IgM) are circulating antibodies in blood and can effectively neutralize pathogenic particles. The neutralizing capability of immunglobulins is highly specific and is directed by previous exposures to infectious pathogens. "We believe that convalescent plasma of recovered SARS patients contain a large amount of antibodies against the SARS virus. We are, therefore, indebted to the Chinese State Council SARS Task Force and Shenzhen Red Cross for their help in the collection of convalescent plasma", said Hong Ji Ling, General Manager of Shenzhen Weiwu Guangming.
In response to the current SARS outbreak, the Chinese State Council Ministry of Science and Technology has commissioned 48 SARS-related projects under the national 863 Programmes. "We are pleased that Advantek Biologics being invited by Shenzhen Weiwu Guangming to participate in one of the 48 National 863 projects and our success demonstrates our strengths in the development of therapeutic proteins," said Bing Lou Wong, Ph.D., CEO of Advantek Biologics. Like other respiratory diseases, SARS may show a seasonal pattern. Vaccine and drug development processes may not be fast enough to cope with recurrences of SARS outbreaks. "As SARS hyperimmune globulins may be an immediate and effective treatment approach, we hope that our efforts could effectively help contain future SARS outbreaks. Our success is setting the standard of Shenzhen-Hong Kong collaborations in the biotechnology industry," added Dr. MB Ali, Chairman of Advantek Biologics and former Assistant Director of Department of Health of Hong Kong.
To fight against possible SARS outbreaks in the future, government officials in the mainland are encouraging various organizations to collect convalescent plasma for the production of SARS hyperimmune globulins. Meanwhile, Advantek has already contacted US higher institutes in public health to investigate the applications of SARS hyperimmune globulins in animal studies and human clinical trials. Grant applications to the US National Institutes of Health are being prepared.
About Hyperimmune GlobulinsConvalescent plasma has been successfully used for treatment of various infectious diseases with no known cures. Mortality rates were drastically reduced in many outbreaks of infectious diseases (example 1: the use of convalescent plasma in the treatment of SARS patients at the Prince of Wales Hospital1; example 2: the use of convalescent plasma in the treatment of Ebola infection2). Immunoglobulins purified from plasma of recovered patients or healthy individuals have been used worldwide for the treatment of paediatric HIV infection, autoimmune diseases, Hepatitis B, rabies, tetanus and other diseases. According to the US National Institutes of Health's Consensus Development Program 1990, "the risks of intravenous immunoglobulins therapy are minimal, and adverse events, which are rare, can often be alleviated by reducing the rate or volume of infusion".
About Weiwu Guangming
Shenzhen WeiWu Guangming Biological Products Company Limited, specialized in the development and production of biological products with 20 years of history, is the largest and most advanced plasma-derived product company in Guangdong Province, China. It was authenticated as one of Shenzhen City Hall and New Technology Enterprises in 1996 and was granted GMP manufacturing status by the State Food and Drug Administration in 1999.
About Advantek
Advantek Biologics Limited, a Hong Kong-based company with two projects sponsored by the Hong Kong Governmentˇ†s Innovation and Technology Commission, is engaged in the R&D, manufacture, marketing and sales of therapeutic proteins. Building on its strengths in the purification of biologics, the Company aims to become a world-class biopharmaceutical company. Proprietary chromatographic technology and Solvent/Detergent (S/D) method are the core protein purification technologies utilized by the Company. The chromatographic technology exclusively (Asia) licensed from Amersham Biosciences (NYSE: AHM) is recognized by regulatory authorities worldwide for the purification of biologics. S/D licensed from the New York Blood Center through Amersham Biosciences is the single most highly validated virus-inactivation method applied to blood derivatives in the world The majority of plasma products manufacturers are currently using S/D method, and more than 34 million doses of S/D-treated products have been administered to patients worldwide. According to New York Blood Center's official records, there have been no reports of hepatitis B, hepatitis C, or HIV transmission associated with S/D-treated products. Validation of SARS virus inactivation is being supervised by Professor John Tam of the Chinese University of Hong Kong.

Disclaimer: The content of this news release does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred. Statements in this news release that are not strictly historical may be forward-looking statements. Actual results may differ from those projected in forward-looking statements due to risks and uncertainties that exist in the company's operations and business environment. The company undertakes no obligation to release publicly the results of any revisions to these forward-looking statements to reflect events or circumstances arising after the date hereof.
